Mimetix® 3D Cell Culture
Mimetix® scaffolds mimics the extracellular matrix by providing an ideal architectural environment to support the growth of cells in 3D. They are created by electrospinning the medical-grade polymer poly(L-lactide) (PLLA) into microfibers, which are highly consistent with regard to fibre diameter and pore size, resulting in excellent reproducibility of cell-based assays.
The Mimetix® scaffold is incorporated into standard SBS footprint well plate frames with bases of superior optical clarity and minimal base distortion. The scaffold depth of 50 µm is thick enough to provide the benefits of 3D cell morphology and behaviour, yet thin enough to allow microscopic imaging
 |
|
|
Advantages
- True 3D micro-environment
- Minimal protocol adaption required to switch from 2D to 3D
- Compatible with industry-standard automated handling and imaging equipment
- High well-to-well and batch-to-batch consistency
- Scaffolds are free from animal-derived products and synthesised using medical-grade polymers.
|
Figure 1. Co-culture of primary keratinocytes and primary dermal fibroblasts in the Mimetix®scaffold after 24 h (fibroblasts in the background, keratinocytes in the foreground). Courtesy of Dr A.J. Bullock, University of Sheffield
Usage and Handling
- Compatible with fluorescent and light microscopy
- Supplied gamma-irradiated in individually-sealed plastic wrapping
- Scaffolds can be coated with materials to facilitate cell adhesion in low serum conditions
Scaffold Specifications:

- Material: medical-grade poly-L-lactide (PLLA), FDA-approved
- Scaffold thickness: 50 µm
- Available fibre diameters: 4 µm (= pores of 15-30 µm)
- Overall porosity: app. 80%
- Non-biodegradable in in vitro applications
- Supplied with low profile lid with condensation rings
-
| Workflow |
2D |
Mimetix® scaffold |
| Preconditioning |
Not necessary |
Wet with 20% ethanol, 2 subsequent washing steps with PBS and leave in culture medium. |
| Coating |
Possible |
As easy as 2D. Larger amounts of the coating agent might be required. |
| Cell seeding |
Varies |
Depends on cell type. Using a similar cell number for seeding than in 2D is a good starting point, but ideally a range of different seeding densities should be tested. |
| Medium exchange |
Easy |
As easy as 2D |
| Plate reader analysis |
Easy |
Fluorescence and Luminescence-based assays: as easy as 2D. Absorbance-based assays: supernatant has to be transferred to a clear plate prior to read-out |
| Flow cytometry analysis |
Easy |
Same as 2D. Potentially longer incubation times/more rigorous shaking with lysis agent needed; please refer to protocol “Optimised cell recovery” for more information”. |
| Microscopic analysis |
Easy |
Confocal fluorescent microscopy: as easy as 2D. Confocal brightfield microscopy: as easy as 2D. Standard optical microscopy: does not work well because of scaffold opacity |
| Ease of Automation |
Easy |
As easy as 2D |
Product Manual
For more information download our Mimetix® product manual featuring: 
 |
|
 |
|
 |
| |
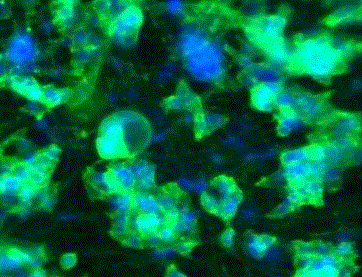
| Cortical oligodendrocyte precursors differentiating into active oligodendrocytes and lay myelin on the Mimetix scaffold in the absence of neurons. Cells stained after 14 days for myelin basic protein (green) and Hoechst (blue). |
|
Percent of oligodendrocyte precursors (NG2) and oligodendrocytes (MBP). More than 600 cells were counted per condition. Mean and standard deviation are shown for three experiments. |
|
Schwann cells grown for 21 days and stained with anti S-100 (purple) and Hoechst (blue). |
 Download High Throughput Myelination Presentation
Download High Throughput Myelination Presentation
Cardiotoxicology

Mimetix Aligned scaffolds improve both structural and functional read-outs in cardiomyocytes, growing 3D cultures of spontaneously beating hiPSC-derived cardiomyocytes (hiPSC-CMs) in 96-well format. hiPSC-CMs grown on such aligned 3D plates showed statistically significantly higher Ca2+ transient rising slope (indicating faster kinetics), lower peak width durations, and lower amplitudes as compared to standard 2D tissue culture plates.
Download presentation (Experimental work performed at Merck, USA)
Toxicology studies
Hep G2 liver cancer cells are often used as model cultures for toxicology studies
in-vitro. Mimetix
® scaffolds have proven to be provide a suitable environment for liver cells:
- Metabolic functions are preserved in Mimetix® for up to 28 days
- CYP activities are similar to those in primary hepatocytes showcasing enzymatic drug metabolism in-vitro
- Phase II enzymes that are involved in solubility of drugs and hormones as well as excretable metabolites display same or increased expression in Mimetix® scaffolds compared to 2D controls

⇨ CYP enzymes activities were assessed using LC-MS by measuring the conversion of their specific substrates in HepG2 culture on Mimetix® scaffold over 28 days. Data from day 21 are shown.
⇩ Cells were seeded at 10000/well in 2D and Mimetix®. RNA was extracted, cDNA was synthesized and qPCR was performed. Gene expression is calculated using the comparative Ct method and is relative to the 2D control on day3 and calibrated to GAPDH. All 4 enzymes either maintain (UGT1A1) or increase (UGT2A1, SULT1A1 and SULT2A1) their expression levels over 21 days.

3D Drug Discovery
Cancer cells grown in a more physiologically relevant 3D cultures have shown increased drug resistance compared to traditional 2D systems. Mimetix® scaffold has been successfully used as a matrix for numerous cancer cell models in 3D drug screening: liver, breast, ovarian as well as lung cancer models.

SKOV-3 were seeded at 5,000 cells per well in 2D and Mimetix®. Cells were grown for 4 days in 2D and 24 days in Mimetix® (optimum schedule for each). Cells were exposed to the drug for 3 days in both schedules. Ovarian cells grown in Mimetix are more resistant to both drugs tested. The resistance is observed when the drug is used at cytostatic concentrations too (below 5mm).
Stem Cell research
 |
 |
| Differentiation of neural stem cells into mature neurons within the Mimetix® scaffold. Cells are stained with DCX (red) Tubulin βIII (green) DAPI (blue). |
Neurite outgrowth on our Mimetix® scaffold. Cells are stained with DAPI (blue), Nestin (green) and GFAP (red). Both images: cell cultures on 12-well plate; courtesy of Lara Stevanato, PhD, ReNeuron. |
Electrospinning Technology

Electrospinning is an established method of producing nanofibres from a wide variety of natural and synthetic polymers. A polymer solution is injected at a constant feed rate though a nozzle or needle which is charged to a high voltage, typically 10 to 30 kV. (more...)
Mimetix
® scaffolds are produced in Class VII cleanroom using state-of-the art electrospinning equipment from IME Technologies and are ISO 13485 certified. Each scaffold batch is checked under a scanning electron microscope (SEM) before shipment to the customer.
Custom Service

AMSBIO offers a custom service for electrospun fibres. These scaffolds can be precisely engineered based customer's choice of material, format, fibre diameter etc. Please find our request form for electrospun scaffolds to place an order for your very own scaffold.
The development process and its documentation comply with ISO 13485 Quality Management System.

21-day culture of hMSCs in the PLLA + collagen/HA scaffold, showing mineral deposition on the surface of osteoblast-like cells at day 14 and 21. Published by researches at University of Malaysia.
Paper available here.
- The extensive design and manufacturing capabilities allow us to develop bespoke electrospun scaffolds from a large variety of polymers and in custom formats.
- Electrospun scaffolds can be designed to mimic the extracellular matrix in terms of its architecture, chemical functionality and mechanical properties, therefore providing an ideal substrate for cell growth, differentiation and ultimately tissue repair.
- Materials can be manufactured into implantable medical devices, coatings to improve the acceptance of implants, or supports for implantation of autologous or allogeneic cells for reconstructive therapy.
- Scaffolds can be made from FDA-approved polymers and formulated to degrade over a defined period in vivo or be used as carriers for therapeutic compounds.
- Bioactive compounds can be incorporated into the fibre structure for targeted release or modifying surface properties.
- We can blend or co-spin various polymers, and can form complex nano-composite materials.
As an example, these electrospun scaffolds are used in a project on corneal surface regeneration led by Professor Sheila MacNeil at the University of Sheffield. The project is supported by the “Wellcome Trust Affordable Healthcare for India” programme and is conducting first-in-man studies in 2015.
(click here for more information).
Related Areas
Recombinant Animal Free Extracellular Matrix 3D Hydrogels
Tools for 3D Cell Culture
Cultrex Basement Membrane Extracts (BME)
2D Animal free, chemically defined extracellular matrices
Detachin Cell Detachment Solution
* AMSBIO is the global source for Mimetix®. Mimetix® is a registered trade mark of and manufactured by Electrospinning Company.