1500A: Isolated Muscle System – Microscope Mountable
型号:1500A
价格:请致电:010-67529703
品牌:aurorascientific

Overview
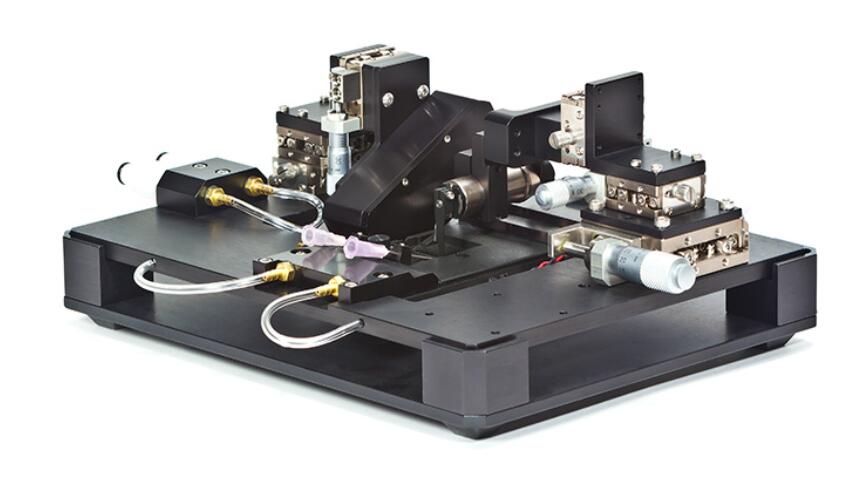
The Isolated Muscle Systems (1500A, 1510A and 1530A) are highly integrated, turnkey systems providing physiology researchers a simple way to control and measure intact muscle and physiological small tissue samples.
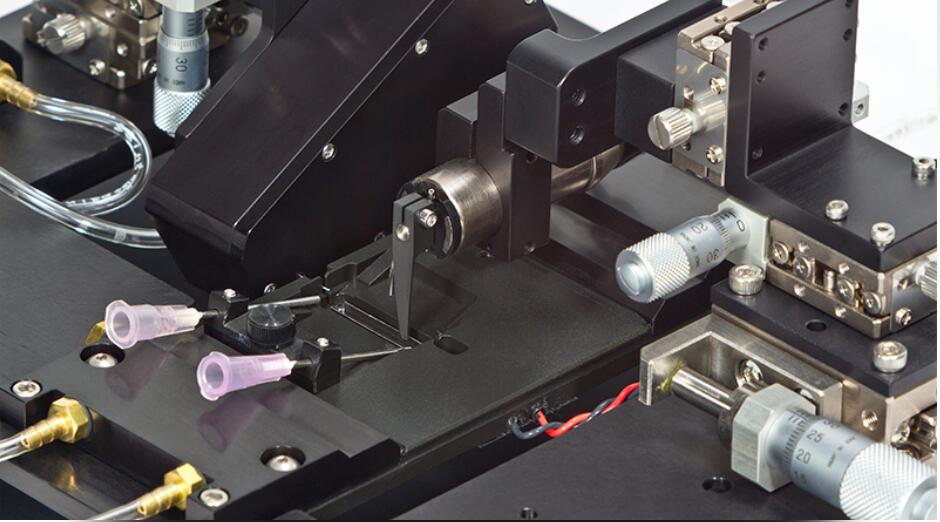
With limitless configuration options, these temperature-controlled systems have the versatility to precisely measure force and length of an array of small tissue types. From engineered tissue constructs to zebrafish the 1500A system is the serious choice for functional muscle measurements. The glass bottom bath chamber with integrated stimulation electrodes and perfusion permit imaging of the sample while maintining tissue viability. The apparatus includes XYZ micrometer stages with built-in mounts for our 400A series of force transducers, high-speed length controllers (322C), and our optional Dual-Mode lever systems (300C). The standard system configuration includes everything required to start testing immediately including apparatus, length controller, force transducer, stimulator, data acquisition hardware and our unique real-time Linux control and analysis software.
The force transducer is attached to the muscle through a unique slot in the end of the bath. This provides an ideal configuration for artificial muscle constructs or oxygen consumption studies that require the chamber to be sealed with an available lid. The 1500A system works with most inverted microscope, allowing integration of sarcomere length and biofluorimetry measurements. Pair your 1500A with our FluoroTrack and HVSL/VSL video sarcomere length modules for force, calcium and sarcomere length measurements all synchronized in real time. These advanced features allow researchers to completely characterize small intact muscle tissue performing all of the standard measurements including twitch, tetanus, fatigue, length-tension, force-frequency, force-velocity, stiffness and work loops.
Signal acquisition and data management is handled by our software, including standardized protocols, which transform complex experiments into simple and straightforward measurement. A stand is included with each system for easy storage and adaptation to a standard stereo or inverted microscope.
Features
- complete Test System with integrated bath apparatus for small intact muscle
- temperature controlled bath plate (sizes from 400?L to 1900?L)
- control and measure both force and length
- compatible with standard and inverted microscopes
- can be integrated with Aurora Scientific 900B Sarcomere Length software (HVSL/VSL)
- resolution as low as 0.01?N
- force ranges from 0.5mN to 1000mN

Case Studies
801C – Ability to Assess Oxygen Consumption/Customization
CHALLENGE
In 2008 Dr. Carter Ralphe at the University of Wisconsin was attempting to measure force and contractility in his tissue constructs in an oxygen sealed environment. Without the time to develop a custom solution, Dr. Ralphe sought help from equipment suppliers. Unfortunately, he wasted a lot of time and money trying equipment from other manufacturers that didn’t work. Although another manufacturer finally refunded his money it took more time and effort to get them to agree to take back their product.
SOLUTION
Dr. Ralphe consulted with Aurora Scientific for the design of a customized oxygen sealing lid that would work with a modified version of our 801C. The redesign allowed the unit to function in oxygen consumption mode or in a regular bath configuration. The customized chamber also allowed easy integration of Aurora Scientific’s industry leading 400 Series transducers which had the sensitivity required for the small and delicate constructs to be tested.
RESULTS
With this instrument, Dr. Ralphe was able to successfully complete his experiments and go on to publish influential results. He now has two units, expanding his research capabilities. Dr. Ralphe has also recommended the device to other researchers who, themselves, have gone on to publish. The 801C-300 has since become essentially a standard modification and a part of our 1500A series of small intact muscle test systems.

Citations
Norden, Diana M. et al. “Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function.” Brain, Behavior, and Immunity 43 (2015): 76-85.
Tangney, Jared R. et al. “Timing and magnitude of systolic stretch affect myofilament activation and mechanical work.” American Journal of Physiology-Heart and Circulatory Physiology 307.3 (2014): H353-H360.
Gharanei, Mayel. “Investigation into the cardiotoxic effects of doxorubicin on contractile function and the protection afforded by cyclosporin A using the work-loop assay.” Toxicology in Vitro 28 (2014): 722-731.
Zuo, Li et al. “Low Po2 conditions induce reactive oxygen species formation during contractions in single letal muscle fibers.” American Journal of Physiology-Regulatory, Integrative and Comparative Physiology304.11 (2013): R1009-R1016.
de Lange, Willem J. et al. “Ablation of cardiac myosin-binding protein-C accelerates contractile kinetics in engineered cardiac tissue.” Journal of General Physiology 141.1 (2013): 73-84.
Tangney, Jared R. “Effects of alterations in sarcomere structure and prestretch timing on cardiac muscle mechanics.” MSc Thesis. University of California San Diego (2012): 1-120.
Zuo, Li, Leonardo Nogueira, and Michael C. Hogan. “Reactive oxygen species formation during tetanic contractions in single isolated Xenopus myofibers.” Journal of Applied Physiology 111.3 (2011): 898-904.
Rhim, Caroline et al. “Effect of MicroRNA Modulation on Bioartificial Muscle Function.” Tissue Engineering: Part A 16.12 (2010): 3589-3597.