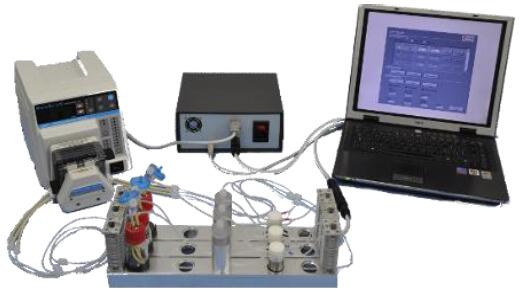
一、意大利品牌意大利多通道三维细胞组织灌注流培养系统
2.特点介绍
(1). 系统配有9个灌流通道,并且每个都相互立
(2). 系统可以双向灌注
(3). 用户可自定义流量,方向和时间
(4). 系统不同的型号支架,用户可根据需求选取,操作灵活
(5). 流速控制范围广泛,0.1 - 6.0 ml/min范围内均可使用,并可j确控制
(6). 应用范围广泛,统te别适合骨、心脏组织圆柱形片段的长期培养
(7). 可以设置多组实验,每次可以单停止一条灌流通路。
(8). 系统配有光学,非侵入性传感器,可以对pH和培养基中的氧浓度进行实时监控。
(9). 锁有的材料都具有生物兼容性且均经过wu菌处理。
3.应用范围
多通道三维细胞组织灌注流培养系统采用可控、双向、间质灌流,通道多达9个,并且相互立,且系统锁有材料都具有生物兼容性且均经过wu菌处理,应用范围广泛,是普通流体研究的里想系统,另外te别适合骨、心脏组织圆柱形片段的长期培养。
应用案例:Effect of Perfusion Culture System on In Vitro Osteogenesis of Human Mesenchymal Stem Cells seeded on Porous Hydroxyapatite
通过激光共聚焦扫描显微镜观察,灌流培养比静态培养得到更好的骨骼组织,且没有改变细胞的活性和扩增能力。
激光共聚焦扫描显微镜检测结果显示灌流培养比静态培养的细胞组织中含有更高的骨钙蛋白的量,产生更均匀、更真实的骨骼组织。
文献:Saino E, Bloise N, Spinelli L,Effect of Perfusion Culture System on In Vitro Osteogenesis of Human Mesenchymal Stem Cells seeded on Porous Hydroxyapatite.
4.主要参数
培养小室wu菌内部放入支架,支架尺寸:
支架尺寸:8 [mm] x h 2 [mm] 灌流直径: 6 [mm]
8 [mm] x h 4 [mm] 灌流直径6 [mm]
10 [mm] x h 2 [mm] 灌流直径 8[mm]
10 [mm] x h 4 [mm] 灌流直径 8[mm]
12 [mm] x h 2 [mm] 灌流直径 10[mm]
12 [mm] x h 4 [mm] 灌流直径10 [mm]
培养基储液瓶:
进口(倒钩接头)
出口(倒钩接头)
采样/培养基换端口(鲁尔锁定接头)
0,22 um过滤器端口(鲁尔锁定接头)
蠕动泵:
流速:0.1 - 6 [ml/min]
管道尺寸:ID 1/32"
多可连接9通道,可有控制器控制
控制器:
配备3种不同的控制器:ACE,基于PC版和基于定时器的版本。
光学传感器:
配备pH和O 2光学传感器,通过串联连接在液流回路。
5.参考文献
(1). Modular perfusion bioreactor--
(2). AutoFeed Automatic medium exchanger
(3). Saino E, Bloise N, Spinelli L, Mantero S, Martinetti R, Imbriani M and Visai L. Effect of Perfusion Culture System on In Vitro Osteogenesis of Human Mesenchymal Stem Cells seeded on Porous Hydroxyapatite. TERMIS EU meeting Granada 2011.
(4). M. Scavone, N. Bloise, E. Saino, L. Spinelli, L. Fassina, S. Mantero, R. Martinetti, L. Visai. Three-Dimensional Perfusion culture of osteosarcoma cell line (SAOS-2) by bidirectional flow. SIB 2010 Camogli
Quasi Vivo细胞灌流培养系统
英国Kirkstall公司生产的创新性细胞培养产品,其Quasi Vivo灌流培养系统可为细胞培养提供持久恒定的流动培养环境,zui大限度模拟体内环境。相比各类传统静态培养系统,Quasi Vivo流动培养系统更大限度模拟了细胞在体内生长环境,其流动培养方式更利于培养基内营养物质的扩散和细胞代谢产物的运输,更有利于复杂细胞模型的构建,尤其适合需要在气液界面进行分化的各类呼吸道上皮细胞的生长。
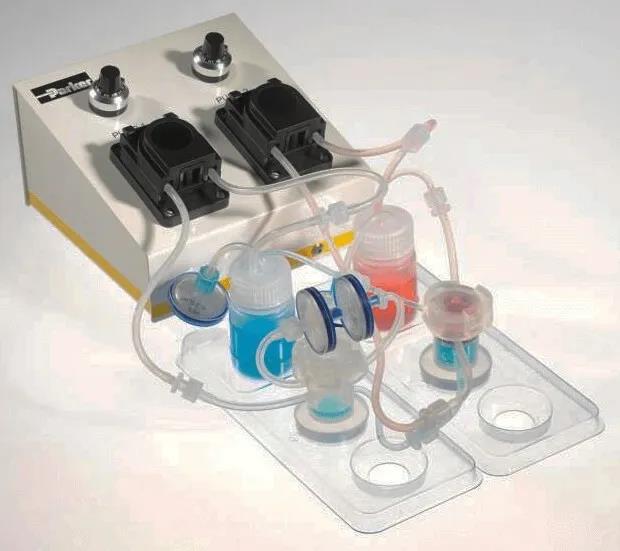

Quasi Vivoquan球应用
quan球使用Kirkstall公司Quasi Vivo灌流培养系统的学术及研究机构已达70+个,遍布美国、英国、法国、瑞典、奥地利、意大利、荷兰、瑞士、日本等。目前Quasi Vivo灌流培养系统已成功用于以下器官模型的培养:
1. 呼吸系统(培养热点)
2. 肝脏
3. 肾脏
4. 心血管
5. 成纤维细胞
6. 糖尿病模型
7. 血脑屏障
8. 脑组织类器官
典型应用例子:
灌流培养
呼吸道上皮细胞的气液界面培养是研究经空气传播的病原体,如SARS等的常用的模型。传统的培养方式是用TransWell在普通培养箱中静置培养。但是此种培养方式wu法模拟培养过程中营养物质和代谢废物在组织内的运输,培养得到的模型通常有各种各样的缺陷,并且所需实验周期较长。
呼吸道上皮细胞的常规transwell静止培养方式

Quais Vivo(QV600)灌流培养系统(腔室+储液瓶+底座+管道+泵等)

而Quasi Vivo灌流培养系统可为细胞培养提供持久恒定的流动培养环境,zui大限度模拟体内环境。研究发现,使用Quasi Vivo系统进行灌流培养与静态培养相比,气液界面培养的呼吸道上皮细胞(正常人气管上皮细胞 Normal Human Bronchial Epithelial Cells,简称NHBE;小气道上皮细胞 Small Airway Epithelial Cells,简称SAE),发育分化速度更快,表现为纤毛分化度更高,纤毛运动更强、粘液产生和屏障功能更强。在灌注下加速分化后,将上皮细胞转移到静态条件下,并添加抗原呈递细胞(APC)以研究其在病原体感染后的功能。(Chandorkar P, et al., Fast-track development of an in vitro 3D lung/immune cell model to study Aspergillus infections. Sci Rep. 2017 7(1):11644. doi: 10.1038/s41598-017-11271-4.)

01、人体内锁有的细胞都需要营养物质和代谢废物的流动

02、肺部气管/支气管和小气道上皮结构精细,进行体外培养模拟体内环境,对呼吸道病原体的研究至关重要

03、采用quan新的灌流培养方式培养呼吸道上皮细胞(采用QV600)
相比使用transwell静止培养(Static Conditions),Quasi Vivo灌流培养系统(Perfused Conditions)中,呼吸道上皮细胞的生长和分化呈现更好状态

04、电镜照片显示,采用灌流培养方式(Perfused conditions)的呼吸道上皮细胞,分化程度更高

05、使用MUC5B染色可以发现,采用灌流培养方式(Perfused conditions)的呼吸道上皮细胞,在培养的弟7天即可分泌大量粘液。用OCCLUDIN染色可以发现,细胞间的紧密连接发育更完善

06、使用WGA染色发现,采用灌流培养方式(Perfused conditions)的呼吸道上皮细胞,纤毛分化度更高

07、测量TEER(经细胞电阻),采用灌流培养方式(Perfused conditions)的呼吸道上皮细胞TEER值更大,代表得到的上皮细胞膜状结构更完整
一、不同细胞,Quasi Vivo型号怎么选?
:使管路上游的细胞培养基成为下游细胞的条件培养基。

流动培养形成含血管的3D心脏组织 | 再生医学
在再生医学领域,怎样培养出含血管的组织,是未来应用能否成功的关键之一。早期的临床试验采用生长因子或细胞注射的方法来修补损伤的心脏,但由于注射细胞造成的炎症反应和局部缺血会在体内造成低氧环境,使得注射的细胞定植率低而死亡率高,不能有效地修复损伤的心脏功能。
Quasi Vivo QV500流动培养系统为接种在明胶支架上的人间充质干细胞(hMSCs)和人心肌祖细胞(hCMPC)提供充足的氧气,促进细胞和营养物质向支架核心内扩散,并能快速有效地排除组织内的代谢废物,促进血管生成,从而形成由血管样和心脏样细胞组成的组织结构密集的适于体内移植的原组织。(Pagliari S, et al. A multistep procedure to prepare pre-vascularized cardiac tissue constructs using adult stem cells, dynamic cell cultures, and porous scaffolds. Frontiers in Physiology. 2014; 5: 210)

Quasi-Vivo流动培养系统 (QV500型)的蠕动泵将培养基从储液瓶泵到两个串联的培养腔室内,并能保持恒定流速(200μl/min),保证多孔明胶支架内层的培养基流动。

构建含血管的3D心脏的实验方案示意图。明胶多孔支架被浸入稀释的Matrigel中,然后转移至内皮分化培养基中。之后将人间充质干细胞接种在支架上,使人间充质干细胞定植在支架培养上并向内皮进行分化,96小时后,将在聚苯乙烯细胞培养板用心脏分化培养基预先定型2周的心脏TNT-GFP人心肌祖细胞接种于血管化的支架上,用QV500流动培养系统在心脏分化培养基中培养7天。

采用上述实验方案,对用QV500培养一周后的共培养结构进行检测,发现在支架上有大量细胞定殖。

QV500流动培养条件下支架内部浸润了大量的血管样细胞(红色)和人心肌前体细胞(hCMPC)衍生的心肌细胞(绿色),而静态培养条件下,细胞大部分分布在支架表面。

免疫组化结果显示通过QV500动态培养可以促进心肌样细胞(GFP,绿色)和内皮样细胞(VCAM-1阳性细胞,红色)向支架内部浸润。

(A) 切片显示QV500流动培养的内皮样细胞(VCAM-1阳性细胞,红色)排列成孔状,形成管状结构,并与心肌样细胞(GFP,绿色)接触。(B)QV500流动培养条件下,支架内广泛的细胞分布导致形成密集组装的多细胞组织,该组织衍生自所用的人间充质干细胞(hMSCs)和人心肌前体细胞(hCMPC)。
总结:在本文中使用的QV500流动培养系统,能增强氧气与营养物质的运输,进而增强工程化心血管组织的活性和功能。
与众不同的Quasi Vivo流动培养系统,让日、美、英、法、瑞士、瑞典等quan球70多个研究机构获得了更强大的细胞培养工具,在包括呼吸系统、心血管系统、肝脏、肾脏、肠道、脑组织类器官,以及糖尿病的研究上更进一步。

流动培养实现血脑屏障三种细胞共培养 | 阿尔茨海默病新模型

血脑屏障(blood-brain barrier, BBB)在中枢神经系统(CNS)的生理和病理中都起着重要的作用。血脑屏障功能异常会引起包括阿尔茨海默症(AD)等许多神经退行性疾病。组成血脑屏障的毛细血管内皮细胞(capillary endothelial cells)、周细胞(pericytes)以及星形胶质细胞(astrocytes)间的复杂的相互作用使得很难在体内确定这三种细胞对神经毒性各自的贡献。

而Quasi Vivo流动培养系统可为体外培养这三种细胞提供在不形成屏障的情况下维持细胞间通讯的zui佳培养环境。Quasi Vivo流动培养系统为未来研究不同类型的血脑屏障细胞在中枢神经系统疾病和细胞毒性试验中的te殊作用提供一个有价值的工具。(Miranda-Azpiazu P, et al. A novel dynamic multicellular co-culture system for studying individual blood-brain barrier cell types in brain diseases and cytotoxicity testing. Sci Rep. 2018; 8(1): 1-10.)

图 1. 单du培养的人星形胶质细胞(A,GFAP阳性)、周细胞(B,α-actin阳性)、血管内皮细胞(C,CD31阳性)以及血管内皮细胞形成的紧密连接(D,ZO1阳性)。

图 2 用Quasi-Vivo QV500培养共享相同的培养基的星形胶质细胞、周细胞和血管内皮细胞的示意图(A),R为储液瓶,P为蠕动泵。连接培养基存储瓶的一个Quasi-Vivo QV500流动培养系统的细胞培养腔室(B)。

图 3 Quasi-Vivo QV500流动培养系统建立的能同时培养三种不同细胞的多细胞共培养体系。

图4 几种流动培养方式示意图:A图为单du星形角质细胞流动培养,B图为单du周细胞流动培养,C图为单du血管内皮细胞流动培养,D图为三种细胞组合后一起流动培养。

图5 用MTT法测细胞活力,与静态培养相比,采用Quasi-Vivo QV500流动培养系统对单du培养血管内皮细胞(HBECs)、周细胞(HBVPs)、星形角质细胞(HAs)(A)或三种细胞共培养(B)的血管内皮细胞的细胞活力有明显升高。

图6 用MTT法测细胞活力,与静态培养(Static)相比,流动培养(Dynamic)的周细胞(HBVPs)会更早受到Aβ25-35(淀粉样蛋白β肽的Aβ25-35片段,用于阿尔茨海默病的造模)的毒害。
总结:本文中研究者利用Quasi-Vivo QV500流动培养系统建立了三种细胞的共培养。这些细胞不接触,通过共享培养基实现细胞间的通信,不形成屏障能更好的研究这些细胞类型单du对不同化合物的响应情况。并且研究者还发现共享相同培养基的星形胶质细胞、周细胞和血管内皮细胞的zui适流速为50 μl/min。
作为创新的细胞培养方法,Quasi Vivo流动培养已经quan球70余家专业机构使用验证,获得了令人侧目的培养效果,在美、英、法、日等多国开展了颇具新意的细胞研究,涉及呼吸系统、肝脏、肾脏、心血管、成纤维细胞、糖尿病模型、脑组织类器官等。
简单介绍
UCUP灌注生物反应器是一种用户友好的工具,用于建立和控制您的3D细胞和组织培养。 Ucup专门设计用于在生命科学相关领域工作的任何科学家或实验室技术人员使用,而不必要求使用生物反应器的任何先前经验。 应用 器官型模型(骨重建,肿瘤微环境) 3D细胞扩增和分化 细胞 - 支架相互作用的研究 细胞外基质相互作用的研究 生成适合临床前实验的3D细胞支架结构
产品描述
Ucup三维灌流培养系统
生成适合临床前实验的3D细胞支架结构 瑞士,Ucup三维灌流培养系统 Organotypic models (Bone remodeling, Tumor microenvironment) 3D cell expansion and differentiation Investigation of cell-scaffold interactions Investigation of cell-extracellular matrix interactions Generation of 3D cell-scaffold constructs suitable for preclinical experimentation Apply instantly your current cell culture concepts and simply let Ucup further extend them by performing the seamless transition to the 3D context. Product Configuration 1x syringe pump 1x rack 1x Starter kit If you are convinced of the benefits that a 3D culture environment can provide,the Ucup bioreactor is the essential tool to conduct with your experiments. For assistance and advice to set up your experiment, do not hesitate to contact CELLEC’s expert team to address your questions. 应用文献: Boccardo and Gaudiello 2016 In this paper, the perfusion-based bioreactor is used for the generation of an adipose mesenchymal stromal cells -based engineered constructs (Title: Engineered mesenchymal cell-based patches as controlled VEGF delivery systems to induce extrinsic angiogenesis, Acta Biomaterials) Cerino 2016 presents an application for engineering an in vitro 3D multi-cellular muscle-like tissue model (Title: Three-dimensional multi-cellular muscle-like tissue engineering in perfusion-based bioreactors, Biotechnology and Bioengineering) Hirt and Papadimitropoulos 2015 demonstrates the importance of perfusion flow in 3D cultures of tumor cells to efficiently mimic functional features observed “in vivo” and to test anticancer compounds (Title: Bioreactor-engineered cancer tissue-like structures mimic phenotypes, gene expression profiles and drug resistance patterns observed in vivo,Biomaterials) Centola 2015 In this study, the perfusion-based bioreactor system is used to improve cartilage digestion, resulting in higher and more reproducible yield of cell populations with high proliferation and chondrogenic capacity (Title: An improved cartilage digestion method for research and clinical applications, Tissue Engineering Part C, Methods) Bao 2015 presents a humanized in vitro model that reduces the need for experimental animal models, while recapitulating key biological events in a periodontal pocket (Title: Establishment of an oral infection model resembling the periodontal pocket in a perfusion bioreactor system, Virulence) Papadimitropoulos 2014 presents an efficient expansion method of mesenchymal stromal cells by direct seeding and culturing fresh bone marrow preparation within the pores of 3D porous scaffold (Title: Expansion of human mesenchymal stromal cells from fresh bone marrow in a 3D scaffold-based system under direct perfusion, PLoS One) Hirt 2014 highlights the potential of perfusion-based models to create 3D tumour microenvironment for cancer immunobiology studies and pre-clinical assessment of innovative treatments (Title: In vitro 3D models of tumor-immune system interaction, Advance Drug Delivery Review) Papadimitropoulos 2013 presents an application/method for seeding open porous rapid prototyped polymeric scaffolds (Title: A collagen network phase improves cell seeding of open-pore structure scaffolds under perfusion, Journal of Tissue Engineering and Regenerative Medicine) Sadr 2012 presents an application/method to generate a decellularized cell-laid extacellular matrix which enhances the biological performance of polymeric materials (Title: Enhancing the biological performance of synthetic polymeric materials by decoration with engineered, decellularized extracellular matrix, Biomaterials) Gueven 2011 presents an application for upscaling osteogenic and vasculogenic grafts (Title: Engineering of large osteogenic grafts with rapid engraftment capacity using mesenchymal and endothelial progenitors from human adipose tissue, Biomaterials) Papadimitropoulos 2011 presents an application for engineering an in vitro bone organ model (Title: A 3D in vitro bone organ model using human progenitor cells, European Cell & Materials) Di Maggio 2011 a review for our approaches to engineering in 3D vitro niches (Title: Toward modeling the bone marrow niche using scaffold-based 3D culture systems, Biomaterials) Santoro 2010 presents an application for upscaling cartilaginous grafts (Title: Bioreactor based engineering of large-scale human cartilage grafts for joint resurfacing, Biomaterials) Scherberich 2007 presents an application for generating osteogenic and vasculogenic grafts (Title: Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity, Stem Cells) Wendt 2006 describes the system for maintaining living uniform tissues in the scaffolds (Title: Uniform tissues engineered by seeding and culturing cells in 3D scaffolds under perfusion at defined oxygen tensions, Biorheology) Braccini 2005 presents an application for generating osteogenic grafts (Title: Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts, Stem Cells) Wendt 2003 describes the principle of the Ucup and its impact on cell seeding (Title: Oscillating perfusion of cell suspensions through three-dimensional scaffolds enhances cell seeding efficiency and uniformity, Biotechnology and Bioengineering)应用:



Features Advantages Benefits Direct perfusion Unifrom cell seeding Uniform tissue Efficient nutrition and waste removal Viable tissue, up to several weeks of culture Physiological conditions (mimicking inetrstitial fluid flow and associated induced shears Physiologically relevant tissue Simple and smart design (patented) Easy and ready to use No previous experience with 3D cell cultures required Minimized manual operations Highly reproducible results Efficient with many cell types Versatile cell and tissue culture models Supple scaffold adaptors Compatible with a wide spectrum of 3D porous scaffolds of various composition, architecture and sizes Access to cell culture medium through valves Suitable to seed and co-culture several cell types, even at different culture time points Possibility to investigate complex cell-cell and cell-extracellular matrix interaction Efficient cell retrieval from scaffolds after culture (with standard enzymatic treatment) Easy cell analyses (cytofluorimetry, gene expression etc.)
The performance of Ucup has been extensively validated and certified by scientific publications in peer-review journals.10x Ucup disposable bioreactor kits 
+ 
+ 
= 
The driving force of the system. It generates the oscillating fluid flow of the cell/medium suspension. It cannot be purchased separately. A rotating rack for easy and correct positioning of Ucup disposable bioreactors. It can also be purchased separately. The central core of the system. It is disposable and it comes with 10x adaptors to fit the specific size of your scaffolds. Scaffolds can also be purchased separately. It provides all what you need to start your 3D cell cultures. Additional accessories (e.g. forceps, syringes) and testing units are also included.