1600A: Permeabilized Myocyte System – Microscope Mountable
型号:1600A
价格:请致电:010-67529703
品牌:aurorascientific

Overview
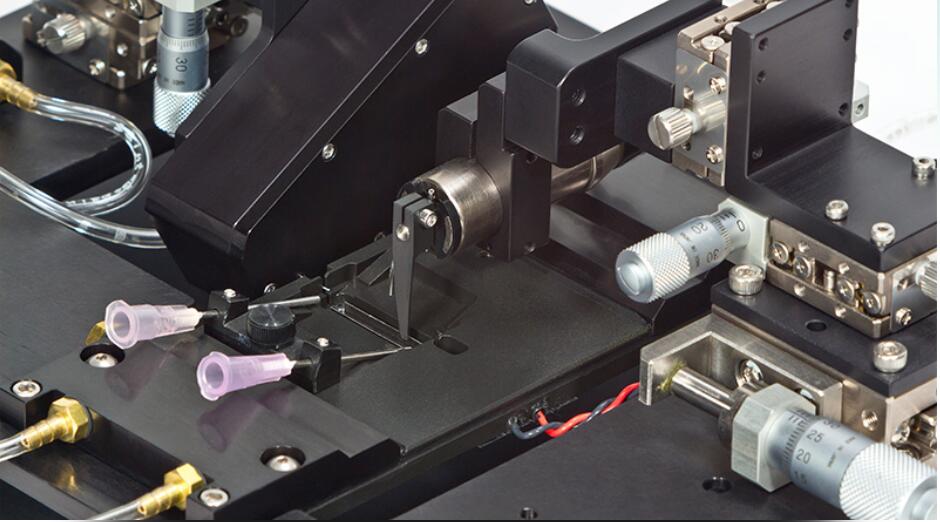
The 1600A Permeabilized Myocyte Test System is designed to simplify the complex testing of contractile properties of skinned myocytes. A large cell mounting area provides ample room for cell attachment. Push button controlled motorized stages simplifies cell attachment to the high-speed servo motor (315C, 322C) and precision force transducer (400 series).
A temperature controlled 8-well bath plate allows for seamless transition of the myocyte between varying calcium concentrations. Direct mounting on an inverted microscope stage allows for observation and sarcomere spacing detection. An optional prism reticule can be included to provide accurate measurement of cell cross section.
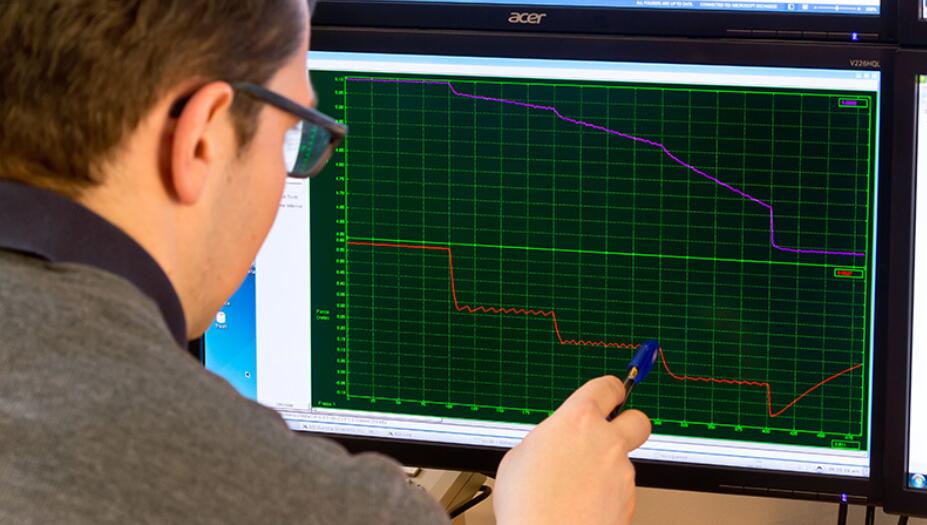
With the 1600A, force, length and sarcomere spacing (with optional HVSL/VSL) can all be controlled and measured easily and repeatedly. This permits measurement of simple force-pCa to more complex mechanical characterization of the cell including kTr, length-tension, force-velocity and stiffness.
The system is truly turnkey, including components for data acquisition and analysis as well. Our Digital controller and Software Analysis package (600A) comes complete with a large library of standardized protocols to make the above characterizations straightforward. Choose the 1600A system for performance, precision and progress.

Features
- complete test system for permeabilized myocytes
- temperature controlled 8-well bath plate (sizes from 400?L to 1900?L)
- large cell mounting area and motorized XYZ micro-positioning stages with computerized control
- control and measure force, length and sarcomere spacing (with optional 900B/901C)
- can be integrated with Aurora Scientific 900B/901C Sarcomere Length Software (VSL/HVSL)
- simple mounting to an inverted microscope
- measure force-pCA, kTR, stiffness, length-tension and force-velocity relationships
- optional optical prism included for measurement of cell depth
Case Studies
1600A – Turnkey Solution Aids Characterization of Cardiac Cell Mechanics in Heart Failure
CHALLENGE
Dr. Jonathan Kirk was working as a postdoctoral fellow in Dr. David Kass’ lab at Johns Hopkins University who had acquired his own custom built equipment for testing mechanics of heart failure in skinned cardiomyocytes. When Dr. Kirk secured a faculty position at Loyola University in Chicago, he did not have the means to fabricate his own custom apparatus and stages to characterize cardiomyocyte function. He also needed to find a way to integrate his own stepper motor for small-scale length changes in single cell preparations while measuring and monitoring sarcomere length.

SOLUTION
Aurora Scientific had worked closely with Dr. Kirk and Dr. Kass previously so we knew we would be able to provide him a complete solution for his new lab. The 1600A Permeabilized Myocyte System was the answer as it included our 803B 8-well skinned myocyte apparatus for various calcium concentrations, 3-axis motorized stages and a complete data acquisition and analysis system. Furthermore, Aurora Scientific provided our 400A series high-resolution force transducer and incorporated our video sarcomere length software (VSL) and camera for measuring and monitoring sarcomere spacing.
RESULTS
The permeabilized myocyte system was provided to Dr. Kirk, which was easily mounted on an inverted microscope and combined with motorized stages to deliver precise control of the transducer and motor for cell attachment and testing. Furthermore, the 8-well bath plate allowed for quick determination of force-pCA in these cells. Additionally, our data acquisition software and VSL enabled full integration of Dr. Kirk’s stepper motor for precise length changes, easily monitored using the VSL camera. The 1600A has provided Dr. Kirk with a system that will allow him to characterize mechanics of heart failure and discover the mechanisms involved, leading the way to the development of potentially ground-breaking treatments for humans with this condition.

Citations
K?tter, Sebastian et al. “Human myocytes are protected from titin aggregation-induced stiffening by small heat shock proteins.” The Journal of Cell Biology 204.2 (2014): 187-202.
Alegre-Cebollada, Jorge et al. “S-glutathionylation of cryptic cysteines enhances titin elasticity by blocking protein folding.” Cell 156.6 (2014): 1235-1246.
Hamdani, Nazha et al. “Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction.” Cardiovascular Research 97.3 (2013): 464-471.
Chung, Eunhee and Gary M. Diffee. “Effect of aging on power output properties in rat skinned cardiac myocytes.” The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 66.12 (2011): 1267-1273.
Horiguchi, Hiroshi et al. “Fabrication and evaluation of reconstructed cardiac tissue and its application to bio-actuated microdevices.” IEEE Transactions on NanoBioscience 8.4 (2009): 349-355.